

Iltis JP, Patel NM, Lee SR, Barmat SL, Wallen WC.Human immunodeficiency virus type 1 antibody in U.S. Evaluation of an indirect immunofluoresence assay for confirmation of Sullivan PT, Mucke H, Kadey SD, Fang CT, Williams AE.The western immunoblot procedure for HIV antibodies and its interpretation. Serological diagnosis of human immunodeficiency virus infection by Western blot testing. The Consortium for Retrovirus Serology.Interpretation and use of the Western blot assay for serodiagnosis of human immunodeficiency virus type 1 infections. LaboratoriesĮxperiencing difficulty obtaining manufactured kits for supplemental testing can contact CDC, telephone 40. Of alternative strategies for HIV diagnostic testing in case shortages of supplemental test kits continue. CDC is collaborating with FDA and other private and public health partners about the evaluation Listed above about increasing production to ensure that sufficient quantities of supplemental test kits will be available for CDC and FDA have contacted all the companies The period during which kits might be in short supply is uncertain. Information about the availability of the oral fluid EIA and WB kits can be obtained

The availability of the OraSure ® HIV-1 collection device is available by telephone, 80, or at Oral fluid samples can be tested further with the supplemental using anĪpproved EIA test kit (Oral Fluid Vironostika HIV-1 MicroElisa) manufactured by bioMérieux, Inc. OraSure ® HIV-1 oral fluid collection device made by OraSure Technologies, Inc. Patient (but not blood or plasma donor) screening for antibodies to HIV can be performed on an oral fluid.Sanochemia provides a self-taught course on performing the HIV-1 IFA and a proficiency Information about the availability of this product is available by telephone, 20, or at Supplemental testing can be performed on serum, plasma, and dried whole-blood spots using theįluorognost HIV-1 IFA kit made by Sanochemia (Vienna, Austria) and distributed by Home Access Health (Hoffman Estates,.
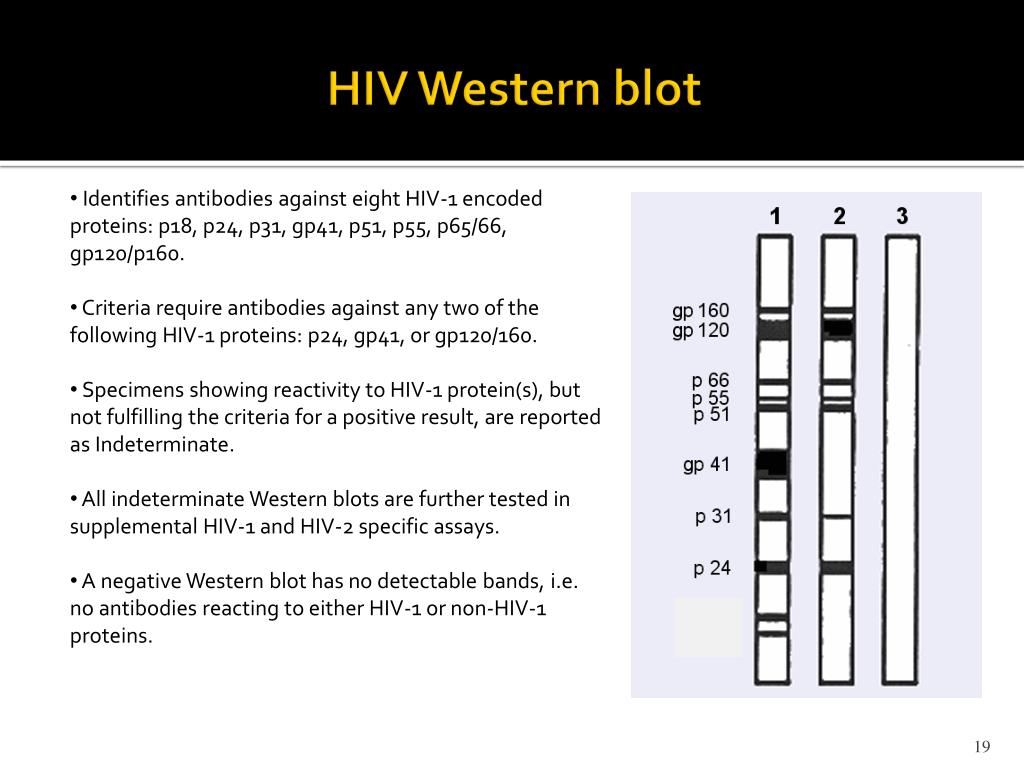
Information about the availability of the test kit is available by telephone, 80, or at Supplemental testing can be performed on serum, plasma, and dried whole-blood spots using the Genetic.HIV antibodies using manufactured test kits approved by FDA: If the Cambridge Biotech HIV-1 Western blot kit is unavailable, three options exist for supplemental testing to detect Persons being tested for HIV might need to be counseled that they might experience delays Some laboratories are experiencing delays in obtaining WB supplemental test kits, and the potential exists for futureĭelays in supplemental testing. This algorithm is used with serum, plasma, dried whole-blood spots, and oral fluid specimens Supplemental tests include the WB test or the indirect immunofluorescence assay (IFA). Specific supplemental test to validate the true-positive EIA results and to prevent notification based on false-positive results that If repeatedly reactive, the specimen is tested with a more Reactive, the EIA is repeated in duplicate on the same specimen. The algorithm for HIV testing in the United States begins with an initial screening enzyme immunoassay (EIA). Not approved for screening and supplemental testing of blood and plasma donors. Samples found reactive for antibodies to HIV-1 in screening tests performed on oral fluids. (Bethlehem, Pennsylvania) and distributed by bioMérieux, Inc., is approved for supplemental testing of oral fluid OraSure ® HIV-1 Western blot kit made by OraSure Technologies,
The other WB test used for these purposes is the Genetic Systems Western blot kit made byīioRad Laboratories, Inc. The Cambridge kit is one of two HIV-1 Western blot (WB) kits licensed by the Food and Drug Administration (FDA)įor supplemental testing of serum, plasma, and dried whole-blood spot specimens obtained for medical diagnosis or bloodĪnd plasma donor screening. (Durham, NorthĬarolina), immediately notified customers that it no longer would be able to distribute the Cambridge Biotech HIV-1 Western blot kit. Stop manufacturing the Cambridge Biotech HIV-1 Western blot kit. On April 17, 2002, Calypte Biomedical Corporation (Alameda, California) announced the company might Testing of human immunodeficiency virus (HIV) antibodies in specimens obtained from either patients or blood and plasmaĭonors. The Public Health Service has become aware of a potential shortage of supplemental test kits used for confirmatory Notice to Readers: Potential Shortage of Supplemental Test Kits For assistance, please send e-mail to: Type 508 Accommodation and the title of the report in the subject line of e-mail. Persons using assistive technology might not be able to fully access information in this file.


 0 kommentar(er)
0 kommentar(er)
